UDI Printers
A unique device identifier (UDI) is a numeric or alphanumeric code that enables unambiguous identification of a medical device. UDIs are generated through a globally accepted device identification and coding system developed by the U.S. Food and Drug Administration (FDA) to enable easier device traceability, improved device monitoring, and increased protection against falsified devices.
These codes allow for quicker identification of flawed devices by healthcare professionals and manufacturers, facilitating faster product recalls, better equipment inventory tracking, and a lower incidence of medical errors. Having access to accurate, consistent, and traceable information about medical devices helps improve patient safety and industry outcomes, especially when it comes to life-supporting devices.
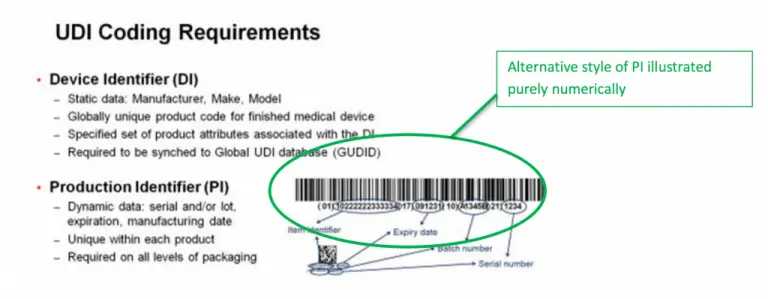
UDI labels consist of the following two parts:
- Device identifier. The device identifier is the fixed section of the UDI label that indicates the specific device. Device identifiers are may only be acquired from FDA-accredited issuing agencies.
- Production identifier. The production identifier usually contains a combination of human-readable text and barcode-friendly marks. This part of the label varies depending on the specific product, but typically includes one or more of the following:
- Batch/lot numbers
- Expiration dates
- Serial numbers
- Production date
- Special coding required for human tissue-based products

According to the FDA, a medical device’s label should include a UDI in both human- and machine-readable forms, and the human-readable form should be written in plain, easy-to-read text. This comprehensive identification system helps address issues related to medical device mislabeling, which can have devastating consequences for both the manufacturer and the end user. For example, administering the wrong drug or using an outdated medical device could lead to patient injury or even death. By allowing for easier and more rapid identification of devices and their attributes, the FDA’s UDI system helps prevent these types of errors.
UDI PRINTERS IN AUSTRALIA#
In addition to establishing new labeling standards, the FDA’s UDI system provides a way for companies worldwide to access critical device information through an expanding international database. Since its establishment in the United States in 2013, other countries have begun implementing UDI system guidelines as a way to improve medical device traceability and safety.
As a result, Australian companies involved in medical-related business in the United States or Europe have likely had to familiarize themselves with UDI packaging regulations over the past several years. As adoption of the UDI system continues to expand globally, medical device manufacturers in Australia should be preparing and upgrading their operations accordingly to avoid any non-compliance issues in the near future. DoraniX is available to help Australian companies get a head start on meeting these new labeling requirements by providing specially configured printers that can meet a range of high-throughput labeling needs.
UDI PRINTERS IN THE UK#
When it comes to the devices used to diagnose, treat, and prevent medical conditions, government regulations are extremely important for verifying product quality and ensuring patient safety. Given the importance of the FDA’s UDI system and its growing global recognition, most medical device manufacturers outside the United States have become familiar with the concept of UDIs.
While this strict labeling system originated in the U.S., UDI implementation for medical devices is now underway in both Europe and the United Kingdom (UK). For UK-based medical device companies in search of printing solutions that will help them comply with these stricter labeling standards, DoraniX provides a variety of robust and customizable industrial printing technologies capable of supporting high-capacity production runs.
UDI PRINTERS IN CANADA#
The FDA’s UDI system plays an important role in minimizing adverse patient outcomes and expensive malpractice lawsuits resulting from medical device mislabeling and misidentification. To remain competitive, Canadian medical device companies doing business in the United States or Europe have had to familiarize themselves with UDI guidelines and the process of making their labels UDI-compliant. Implementing these new regulations often involves replacing outdated printing technologies with newer systems capable of meeting demands for higher print quality and increased outputs. DoraniX can help Canadian-based companies prepare for UDI compliance with our custom printing solutions designed for a range of medical packaging applications.
BENEFITS OF DORANIX UDI PRINTERS#
ThermaPrint64 On DemandAs the UDI system continues to expand in scope, it is becoming increasingly important for medical device companies worldwide to take steps toward implementing UDI-compliant labeling into their medical packaging operations. DoraniX’s heavy-duty ThermaPrint 64 series offers a high-quality solution for UDI-related data printing and barcoding. These versatile printers can be uniquely configured to meet a variety of medical labeling needs, including the printing of variable data directly onto medical-grade Tyvek. UDI information can be easily printed onto medical pouches, surgical lids, bags, and other Tyvek surfaces.
Featuring both Ethernet and USB ports, our ThermaPrint 64 series can be conveniently integrated into existing networks. Other advantages of this series include:
- Unmatched printing speed and efficiency. The near-edge 305 DPI printhead used in the ThermaPrint 64 series combines optimum print quality with high speed and durability, while the ribbon-saving mechanism reduces ribbon consumption and extends life by 90%.
- Customizable configurations. The ThermaPrint 64 series can be customized to meet the specific requirements of each unique printing application, accounting for substrate thickness, surface roughness, adhesives, coatings, and other surface-related factors. These printers can also be configured with 4-6 motors, depending on the application’s power demands. In addition, expanded collection spaces and powered stackers can be integrated to accommodate high-capacity production runs. For flat-sheet packaging needs, these printers can be configured as roll-to-sheet systems through the integration of industrial cutters and roll feed modules.
- Exceptional memory. With a 64-bit processor and 16 MB memory, the ThermaPrint 64 series can handle complex, high-throughput jobs and is compatible with most types of software.
- Compact size. Each printing system can be configured and sized to meet specific labeling purposes. Designed to be compact, most ThermaPrint 64 systems are 26″ in width, 14″ in height, and 14″ in diameter, with weights ranging from 55-86 lbs.
LEARN MORE ABOUT DORANIX PRINTING SOLUTIONS FOR UDI LABELING#
When it comes to medical devices, proper labeling plays an indispensable role in providing essential product information to consumers and enabling improved traceability across the supply chain. As part of a push for greater safety in the medical device industry, the FDA developed the UDI system to improve and streamline the device labeling process. Global UDI implementation has become important for enhancing patient safety, improving post-market product surveillance, and supporting medical device innovation.
DoraniX designs and manufactures a range of printing solutions to help clients in the medical device manufacturing field meet increasingly stringent labeling regulations. Our high-quality, innovative printing technologies include the ThermaPrint 64 series, which is well-suited for printing UDI coding and other types of information directly onto medical device packaging. Working closely with each client to understand their specific labeling requirements, we provide tailored printer configurations that enable seamless integration into existing operations. Regardless of your location, we aim to make the transition to stricter medical device labeling as easy as possible.
We are confident in our ability to provide exceptional, well-targeted printing solutions for a range of demanding labeling applications. To learn more about our printer options and how they can be customized to fit your requirements, contact us. You can also refer to our complete guide to UDI and how unique device identification currently works,